
Commissioning, Qualification & Validation (CQV)
Commissioning, Qualification & Validation (CQV)
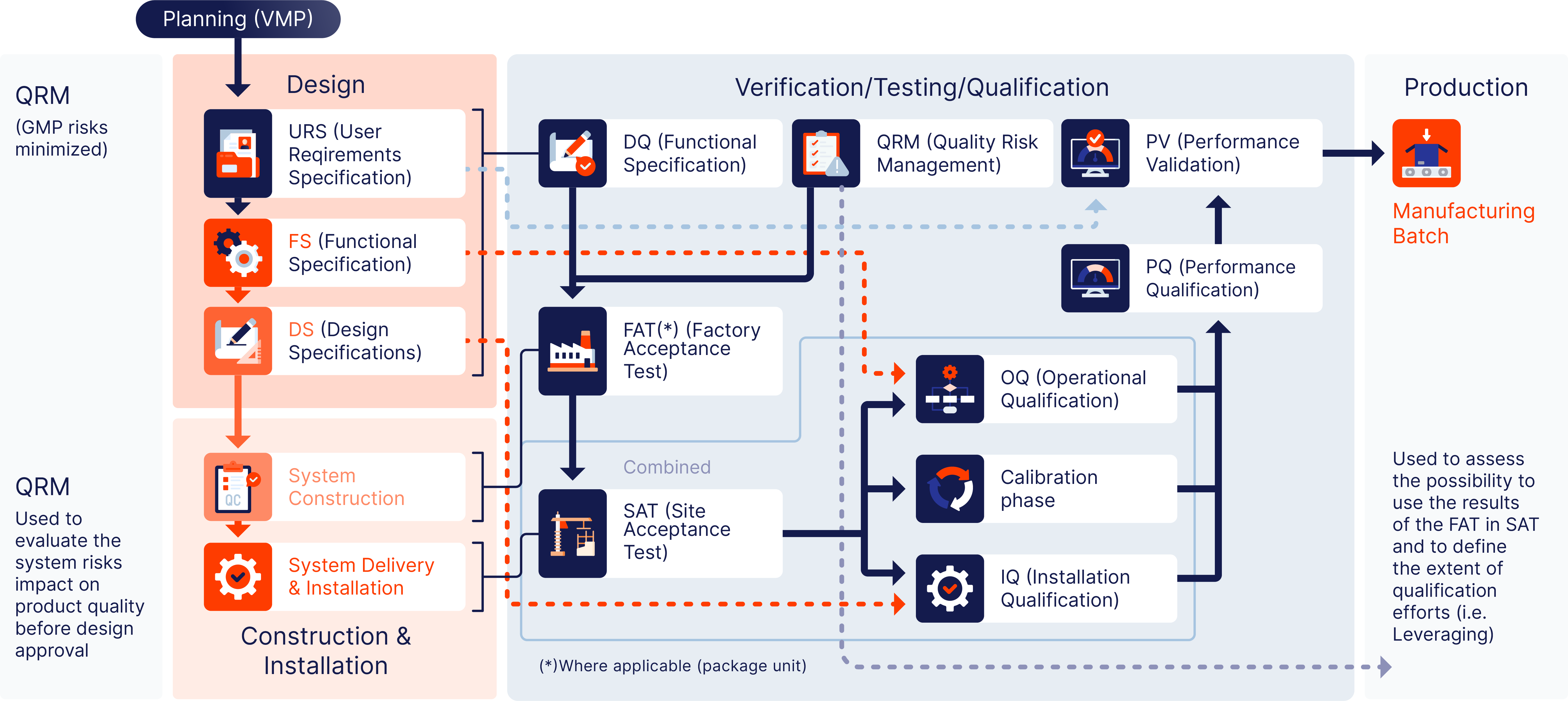
DOC supports pharmaceutical and life science companies in ensuring that their facilities and equipment meet all regulatory, functional, and operational requirements — even before the system is installed.
Validation Lifecycle approach
Get in touch with us!

Manfredi Curtò
DOC | Sales & Business Development Manager

