What is Blood plasma fractionation?
Blood plasma fractionation is a complex, precise process that begins with collecting whole blood and separating its components, including red blood cells, white blood cells, platelets and plasma, before further isolating plasma proteins for therapeutic use. After plasma collection, the product is frozen at ultra low temperatures until ready for fractionation. The first and most critical step is thawing: frozen plasma must return to liquid form quickly and uniformly to avoid thermal stress that could compromise protein integrity. Proper thawing protocols maintain the raw material’s safety, potency and efficacy and ensure compliance with regulatory standards.
Once thawed, plasma protein fractionation proceeds through the progressive addition of ethanol to precipitate specific proteins such as clotting factors, albumin, immunoglobulins, fibrinogens, enzymes and antibodies, followed by centrifugation to collect each fraction. These plasma derived products are essential for treating immune disorders, hemophilia, neurological diseases and other conditions.
Olsa, part of Masco Group, has leveraged years of expertise to develop the ThawMX®, a proprietary thawing tank that delivers thawing rates twice as fast as traditional systems while maintaining strict temperature control. Its design integrates Transfer Panels for seamless intermediate batch handling and cleaning in place (CIP) of vessels and pipelines, along with Temperature Control Units (TCUs) to monitor and regulate temperatures throughout the process. This combination of high performance and safety safeguards the quality of plasma proteins from thawing through fractionation.
Masco Group applies decades of engineering innovation to support every stage of blood fractionation. From modular collection centers and thawing solutions like the ThawMX® through fill finish operations and digital optimization, Masco Group adhere to industry best practices and continually refine equipment and protocols to ensure plasma derived biologics meet the highest standards and deliver life saving therapies to patients worldwide.
Overview of the Blood Fractionation Process
Plasma collection
Plasma collection begins in dedicated centres where whole blood is drawn from screened donors under strict aseptic conditions. Anticoagulants such as citrate are added immediately to prevent clotting. The collected units are then processed by centrifugation, which separates the heavier cellular components (red cells, white cells and platelets) from the lighter plasma fraction. The clear plasma is pooled, tested for infectious agents and quality parameters, then frozen rapidly at ultra low temperatures to preserve protein function until further processing.
Cold ethanol fractionation (Cohn process)
The classic Cohn method uses precise control of temperature, ethanol concentration and pH to selectively precipitate plasma proteins in sequential stages. In the earliest steps, mixtures at lower ethanol concentrations (around 8 percent) and moderate cold (approximately −3 °C) precipitate high molecular weight proteins such as fibrinogen and certain globulins. After removal of these fractions by centrifugation, ethanol concentration is increased (up to 40 percent) and the temperature is lowered (to −10 °C) while the pH is adjusted near albumin’s isoelectric point. This causes albumin to come out of solution with high purity. Each precipitate is collected, washed to remove residual ethanol and redissolved for subsequent purification.
Advanced purification
To achieve pharmaceutical grade purity and safety, plasma fractions undergo additional steps. Ultrafiltration and diafiltration concentrate proteins and exchange buffers. Chromatography techniques ion exchange to separate based on charge, affinity to bind specific molecules, and size exclusion to distinguish by molecular weight remove remaining impurities. Throughout these stages, virus inactivation and removal treatments are applied, including solvent detergent treatment to disrupt lipid enveloped viruses and nanofiltration to physically clear small viral particles. Modern facilities increasingly use single use fluid paths and continuous chromatography platforms, coupled with automated buffer preparation, to maximise yield, minimise cross contamination risk and streamline validation.
Final processing and fill finish
After purification, each protein solution is subjected to stringent viral safety steps such as low pH incubation or heat treatment to inactivate residual pathogens. The bulk drug substance is then sterile filtered through sub 0.2 micron membranes and formulated with stabilisers or excipients as required. Aseptic filling into vials or prefilled syringes takes place in Grade A cleanrooms under automated filling lines, ensuring accurate dosing and minimal human intervention. Units are labelled, batch tested for potency, purity and sterility, then packaged with tamper evident seals and barcodes. Final quality control includes release testing against pharmacopeial standards before distribution to clinics and hospitals.
Types of Blood Derivatives
| Derivative | Description and Use |
| Albumin | Maintains oncotic pressure; used in trauma, burns, surgery. |
| Immunoglobulins | Pooled antibodies for immune deficiency and autoimmune disorders. |
| Clotting factors | Factor VIII (haemophilia A), Factor IX (haemophilia B), prothrombin complex for bleeding disorders. |
| Fibrinogen | Treats congenital deficiencies and massive haemorrhage. |
| C1 inhibitor | Management of hereditary angioedema. |
| Alpha 1 antitrypsin | Treats emphysema in patients with genetic deficiency. |
Each derivative undergoes tailored purification and viral inactivation to meet strict potency and safety criteria.
Masco Group’s Capabilities
Masco Group provides a turnkey solution across the entire plasma fractionation value chain:
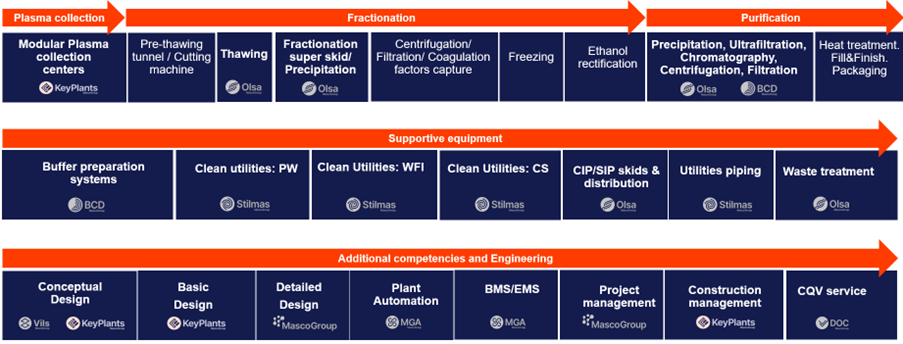
Typical Process Flow: Blood Plasma Fractionation Process at Masco Group
1. Plasma Collection
- Modular Plasma Collection Centers: Designed and equipped by Masco Group’s company, KeyPlants, to ensure optimal efficiency and safety in plasma collection.
2. Fractionation
- Thawing: Utilizing advanced technology from Masco Group’s Olsa, we ensure continuous, rapid and consistent thawing with accurate temperature control, which is crucial for maintaining protein integrity.
- Fractionation Super Skid/Precipitation: Engineered by Olsa to manage ethanol addition precisely for protein precipitation ensuring high process yield.
- Centrifugation/Filtration/Coagulation Factors Capture: These critical steps ensure the isolation and purification of specific plasma components.
- Freezing and Ethanol Rectification: Final processing steps to stabilize and store the plasma components for further use.
3. Purification
- Protein Dissolution: Thanks to Olsa expertise in thawing, we ensure quick and consistent dissolution of proteins in this first critical step of purification
- Advanced Purification Processes: Including precipitation, ultrafiltration, chromatography, centrifugation, and filtration, handled by Masco Group’s companies BCD and Olsa, to ensure the highest purity and functionality.
- Heat Treatment, Fill & Finish, Packaging: Final preparations for the plasma components to be ready for medical use, ensuring safety and efficacy.
4. Supportive Equipment
- Buffer Preparation Systems: Provided by BCD to prepare solutions that aid in plasma processing.
- Clean Utilities: Managed by Stilmas, including purified water (PW), water for injection (WFI), and clean steam (CS), vital for maintaining a sterile processing environment.
- CIP/SIP Skids & Distribution: Designed by Olsa to facilitate the cleaning and sterilization of process equipment and piping.
- Utilities Piping: Ensured by Stilmas to provide necessary services throughout the facility.
- Waste Treatment Systems: Developed by Olsa to handle and treat waste products from the fractionation process safely and efficiently.
- Transfer panel and valve block array: provided by BCD, Olsa to ensure maximum flexibility in fractionation and purification processes • Temperature control units: provided by Olsa to grant accurate temperature control for thawing and precipitation tanks, but also for third party centrifuge systems
5. Additional Competencies and Engineering
- Conceptual and Basic Design: Carried out by Masco Group’s VILS and KeyPlants to establish the foundational design parameters and overall layout of the fractionation facility.
- Design: Executed by Masco Group to finalize all aspects of the engineering design.
- Plant Automation and Control Systems: Implemented by Masco Group Automation (MGA) to enhance process control and ensure compliance with the highest standards and regulatory compliance.
- Building Management Systems/Environmental Monitoring Systems (BMS/EMS): MGA integrate the automation layer with BMS/EMS.
- Project and Construction Management: Managed in-house by Masco Group and KeyPlants to ensure projects are delivered on time, within budget, and meet all regulatory requirements.
- Commissioning, Qualification, and Validation (CQV) Service: Provided by DOC to verify and validate the facility and equipment performance.
- Digital Solutions: comprehensive digital solutions by Masco Group Automation (MGA), powered by AI, designed to optimize pharmaceutical operations, consumption and overall efficency
*) In the example above, where no Masco Group companies are mentioned, the activities will be managed by a trusted third-party contractor, with Masco Group serving as the main contractor.
Our Unwavering Commitment to Blood Plasma Fractionation
Blood plasma fractionation is more than a service at Masco Group, it’s our specialty. We are committed to ensuring that each facility we design and equip meets rigorous standards of safety, efficacy, and quality, making a significant impact on patient lives worldwide.
To see one of our reference cases please visit this link: MasterPlasma, Plasma Fractionation Facility | Keyplants
Contact Us
Discover the Masco Group difference in blood plasma fractionation and purification. Contact our experts today for a detailed consultation or visit our facilities to witness our commitment and expertise firsthand, and learn how we can tailor the fractionation and purification processes to meet your specific needs.